Chemical Reactions is an ultimate tool for balancing chemical reactions, combining of the multistep chemical equations, investigation of complex reaction mechanisms and evaluation of thermodynamic parameters, such as enthalpy (heat release/absorption) and equilibrium constants.
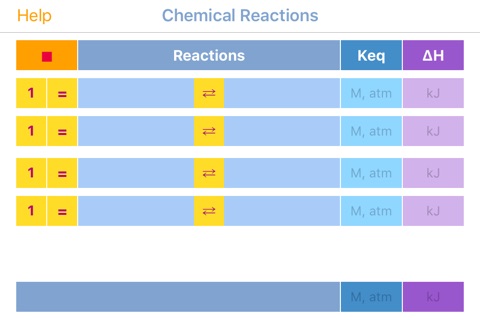
The application interface consists of four reaction rows; each one of which is provided with the following functional elements:
Multiplier: Integer value, defining how many times this reaction proceeds during the process. Recalculates stoichiometric coefficients, enthalpy and equilibrium constant after pressing done button in the keyboard! Should be changed only after balanced reaction is set. To reset, delete all digits in the field and press done button.
Balance button (=): Balances the provided reaction. Ignores the already provided coefficients. Application automatically present final reaction after pressing done button in the keyboard! Does not intended to work with redox, radical and electron flow reactions, instead consider Electrochemistry app!
Reaction: The field to type in reactants and products divided by =. Charge or unpaired electrons (in free radicals), if exist must be provided as well, separated by coma: Na,+ ; R,: . Application automatically presents final reaction after pressing done button in the keyboard! Swipe the field from left-to-right to reverse the reaction. Enthalpy and equilibrium constant are adjusted on the fly. Useful for investigation of complex reaction mechanisms. Application automatically presents final reaction after pressing done button in the keyboard!
Keq: Equilibrium constant for particular row. Automatically recalculated after change of multiplier. Application automatically recalculates final values after pressing done button in the keyboard!
Enthalpy: Should be negative for exothermic reaction (heat release) and positive for endothermic reaction (heat absorption). Automatically recalculated after change of multiplier.
Application automatically recalculates final values after pressing done button in the keyboard!
Important points
Application supports state indexes: (g), (s), (l), (aq) – all are lowercase only, placed at the end of compound formula. Considered to be a crucial part of a molecule, so same molecules of different states appearing on the opposite sides of equation are not cancelled!
Charges of molecules or unpaired electrons must be placed at the end of molecule and separated by coma. They are also considered to be a crucial part of a molecule, so same molecules that have different charges, appearing on the opposite sides of equation are not cancelled!
For further details refer to www.volard.wordpress.com